Resources
Glossary
Passivation
Passivation is a chemical process used to improve the corrosion resistance of metals, especially stainless steel and other alloys. Even though stainless steel already contains chromium, which naturally forms a thin oxide layer that protects it from rusting, the surface can still be contaminated with free iron or other impurities during machining, forming, or handling. These contaminants can make the metal more vulnerable to localized corrosion if not removed.
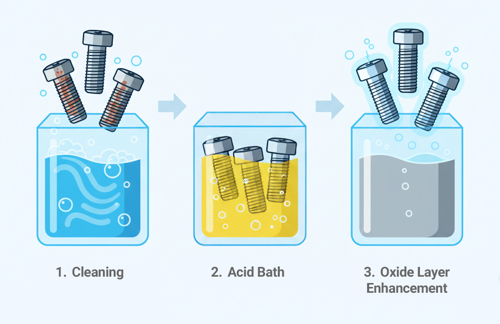
The passivation process typically involves immersing the metal in an acid solution, most commonly nitric acid or citric acid. The acid removes free iron and other foreign materials from the surface without significantly attacking the underlying metal. Once the surface is cleaned, oxygen from the air reacts with the chromium in the steel to form a stable, uniform, and self-repairing chromium oxide layer. This thin passive film serves as a barrier that greatly reduces the rate of corrosion.
In the context of fasteners, passivation is essential because bolts, nuts, and screws are often exposed to moisture, chemicals, or harsh environments where corrosion resistance is critical. By undergoing passivation, stainless steel fasteners gain improved durability and maintain their structural integrity and appearance over longer service lives.
Passivation has advantages, including enhancing corrosion resistance, extending component life, and improving cleanliness without altering part dimensions or strength. However, it also has limitations: it cannot restore heavily corroded parts, requires strict control of process conditions, and is only effective on alloys that contain sufficient chromium to form the protective oxide layer.
Article: Understanding Passivation in Stainless Steel Fasteners
