
Understanding Passivation in Stainless Steel Fasteners
Understanding Passivation in Stainless Steel Fasteners
Stainless steel fasteners are valued for their high strength, durability, and exceptional resistance to corrosion. They’re used across a wide range of industries and can be found in electronics, food processing equipment, medical devices, home appliances, and large-scale construction equipment.
Although stainless steel is often considered a maintenance-free material, certain manufacturing processes can weaken its surface protection. During forming, machining, or handling, microscopic contaminants and imperfections can leave the material vulnerable to oxidation. To maintain the material’s protective surface, stainless steel must undergo a finishing process called passivation, a chemical treatment that restores and strengthens the metal’s natural corrosion barrier.
Passivation is an important part of protecting stainless steel, but to understand why it’s necessary, it helps to first look at how the material itself is made and how its protective surface layer naturally forms.
How Stainless-Steel Alloys Are Made
Stainless steel is made primarily from iron and chromium, often with additional elements such as nickel and molybdenum depending on the grade and application. The chromium content, at least 10.5% by mass, is what gives stainless steel its inherent ability to resist rust.
In production, these elements are melted together in industrial furnaces, refined, and cast into the desired form. When the solidified alloy is exposed to oxygen, the chromium at the surface reacts with it to form a thin, invisible layer of chromium oxide. This passive film acts as a stable, self-healing barrier that prevents rust from forming on the steel underneath. This naturally formed layer is also responsible for stainless steel’s recognizable, bright finish, giving it a smooth and reflective appearance.
While stainless steel’s chromium oxide film provides strong corrosion resistance, it can be compromised during manufacturing. When that happens, the material needs help restoring its full protective qualities. That’s where passivation comes in.
Passivation Explained
It’s important to note that passivation isn’t a coating or an applied finish. It’s a chemical process that removes surface contaminants and reinforces stainless steel’s natural ability to resist corrosion.
As mentioned earlier, stainless steel can pick up trace amounts of iron from cutting tools or other equipment. These tiny surface deposits don’t bond with the alloy and are more prone to rusting. This contamination is known as free iron. If left on the surface, free iron can oxidize when exposed to moisture, creating rust that spreads and weakens the surrounding material.
Passivation removes these contaminants and restores the stainless-steel surface to its clean, corrosion-resistant state. Here’s how it works in practice:
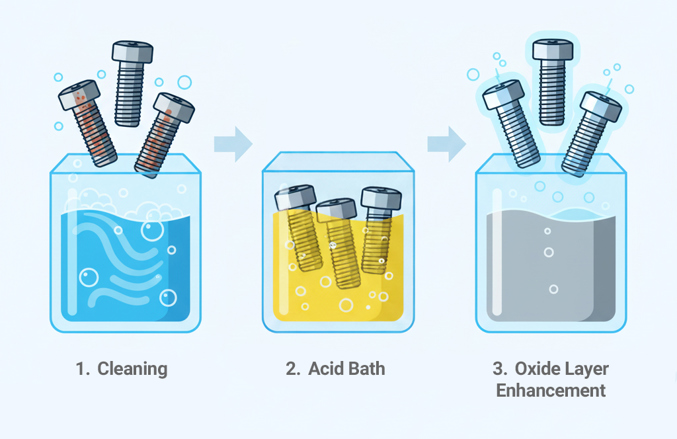
- Cleaning: Fasteners are thoroughly cleaned using an alkaline detergent or solvent cleaner to remove machining oils, lubricants, and other residues left from manufacturing. In some cases, parts may also be rinsed with water or ultrasonically cleaned to ensure the surface is completely free of contaminants before passivation begins.
- Acid Bath: The fasteners are immersed in a nitric or citric acid solution. The acid selectively dissolves and removes free iron and other contaminants while leaving the stainless steel itself untouched.
- Oxide Layer Enhancement: With the surface clean and free of impurities, the acid bath promotes the rapid formation of a fresh chromium oxide layer. The result is a uniform, stable, and dense film that enhances the material’s corrosion resistance.
After treatment, the fasteners are thoroughly rinsed with deionized or distilled water to remove any remaining acid and contaminants. They’re then dried in a clean environment, typically using warm air or controlled heat, to prevent water spots or surface oxidation. The result is a stainless-steel fastener with a clean, passive surface and optimized corrosion resistance. You can’t see the effects of passivation, but they make all the difference in performance and longevity.
Why Passivation Matters
Passivation might be one of the final steps in manufacturing stainless steel fasteners, but it’s also one of the most important. By removing surface contaminants and restoring the protective chromium oxide layer, passivation ensures stainless steel delivers the corrosion resistance it’s designed for. It’s what keeps fasteners from seizing, staining, or failing prematurely in service, protecting both the product and the assemblies they hold together.
At Earnest Machine, we understand how manufacturing details impact the fasteners you depend on. We’re here to help you source quality products efficiently and keep your business moving forward. For questions about product availability or support, contact us at 1-800-327-6378 or email [email protected].
