Resources
Glossary
Chloride Stress Corrosion Cracking
Chloride Stress Corrosion Cracking (Cl-SCC) is a type of environmentally assisted cracking that occurs when certain metals—especially austenitic stainless steels—are exposed simultaneously to tensile stress and chloride-containing environments (such as saltwater, seawater mist, or de-icing solutions). It is one of the most insidious forms of corrosion because it can cause sudden and catastrophic failure with little or no visible warning.
This phenomenon arises from a combination of three key factors: a susceptible material, a chloride-rich environment, and tensile stress (either from external loads or residual stresses from welding, cold working, or assembly). When all three are present, microscopic cracks can initiate at points of stress concentration—such as welds, notches, or pits—and then grow over time under relatively low stress levels.

Chloride ions are particularly aggressive because they can penetrate and locally destroy the passive oxide film that normally protects stainless steels. Once this film breaks down, localized anodic dissolution begins at the crack tip, while the surrounding metal remains cathodic. The crack then propagates through repeated cycles of film breakdown and repassivation, driven by the tensile stress. This process often produces branching, brittle-looking cracks that can penetrate deep into the metal.
Cl-SCC is most severe at elevated temperatures (typically above 50°C or 120°F) and in environments with high chloride concentrations, such as marine atmospheres, chemical plants, and desalination systems. It is a major concern in stainless steel piping, pressure vessels, heat exchangers, and fasteners.
Prevention strategies include reducing tensile stress (via stress relief or redesign), controlling chloride exposure, using more resistant materials (such as duplex or ferritic stainless steels, or nickel-based alloys), and maintaining lower operating temperatures.
In summary, chloride stress corrosion cracking is a localized, brittle fracture mechanism caused by the combined action of tensile stress and chloride attack on metals—most notably stainless steels—resulting in rapid, often unexpected failure even in materials that otherwise appear corrosion-resistant.
Corrosion (Metallic Corrosion)
Metallic corrosion is the gradual degradation of a metal through a chemical or electrochemical reaction with its environment, resulting in the conversion of the metal into more stable compounds such as oxides, hydroxides, or sulfides. In essence, it is the reversion of refined metal back to its natural, ore-like state—for example, iron rusting back into iron oxide.
Corrosion occurs because most metals exist in a high-energy, unstable state after refining from their ores. When exposed to oxygen, water, acids, salts, or other reactive substances, they naturally tend to return to a lower-energy, oxidized form. The most common and damaging type of metallic corrosion is electrochemical corrosion, which involves the flow of electrons between anodic (oxidizing) and cathodic (reducing) sites on the metal’s surface.
Here’s how it happens:
1. At the anode, metal atoms lose electrons and form positive metal ions — for example:
Fe → Fe²⁺ + 2e⁻
This is oxidation, and it’s the site of metal loss.
2. At the cathode, a reduction reaction occurs, often involving oxygen and water, such as:
O₂ + 2H₂O + 4e⁻ → 4OH⁻
3. The electrons released at the anode travel through the metal to the cathode, completing the circuit. The metal ions produced at the anode can react further with oxygen or water to form corrosion products like iron oxide (rust).
Environmental factors such as humidity, temperature, pH, and salt concentration greatly influence the rate of corrosion. For example, saltwater accelerates corrosion by increasing electrical conductivity, while acidic environments promote metal ion dissolution.
There are several types of metallic corrosion, including:
- Uniform corrosion, where the surface degrades evenly (common in steel rusting).
- Galvanic corrosion, which occurs when two dissimilar metals are electrically connected in an electrolyte, causing one to corrode preferentially.
- Pitting corrosion, a localized attack that produces small but deep holes.
- Crevice corrosion, which occurs in shielded or tight areas where oxygen levels are low.
- Intergranular and stress corrosion cracking, which occur along grain boundaries or under tensile stress in corrosive environments.
Prevention and control of corrosion rely on barrier coatings (paint, plating, galvanizing), cathodic protection, corrosion-resistant alloys (like stainless steel, titanium, or Inconel), and environmental control (e.g., desiccation or pH adjustment).
Corrosion Nucleation
Corrosion nucleation is the initial stage in the corrosion process where the very first microscopic sites of corrosion begin to form on a metal surface. At this stage, the metal is still mostly intact, but localized regions—often defects, impurities, scratches, grain boundaries, or inclusions—become energetically favorable spots where corrosion reactions can start.
When a metal is exposed to an aggressive environment (such as moisture, salts, acids, or other electrolytes), small electrochemical cells form on the surface. Certain areas act as anodic sites, where metal atoms begin to lose electrons and dissolve, while surrounding areas act as cathodic sites, where reduction reactions occur. This uneven distribution of activity causes specific points to become the “nuclei” or seeds of corrosion.

Nucleation is critical because it determines how corrosion will progress. If the nucleation sites remain few and isolated, corrosion may advance slowly and be easier to manage. But if many nuclei form across the surface, corrosion spreads more rapidly, leading to generalized damage. In other cases, nucleation may be highly localized, giving rise to pitting corrosion, crevice corrosion, or other aggressive localized attack that can cause sudden failures.
The factors influencing corrosion nucleation include the material’s microstructure, surface condition, applied stresses, environmental chemistry, and temperature. Understanding and controlling this stage is important in corrosion prevention because once nucleation sites establish themselves, corrosion tends to accelerate and propagate outward.
Crevice Corrosion
Crevice corrosion is a localized form of corrosion that occurs in narrow, confined spaces where oxygen and other environmental conditions become trapped, creating a chemical imbalance. These spaces—called crevices—can exist between two metal surfaces or between a metal and a nonmetal, such as under washers, gaskets, fastener heads, lap joints, or even beneath dirt and deposits.
The problem begins when a small volume of electrolyte (like saltwater or moisture) enters the crevice. Inside the tight space, oxygen becomes depleted because it cannot easily circulate. Meanwhile, the surrounding surface outside the crevice remains exposed to oxygen. This difference in oxygen concentration creates an electrochemical cell: the area inside the crevice becomes anodic (where metal dissolves), and the area outside becomes cathodic (where reduction occurs). As a result, the trapped electrolyte inside the crevice becomes more acidic and chloride-rich, accelerating metal dissolution.

Over time, this process leads to pitting, discoloration, and material loss concentrated within the crevice. It is particularly problematic because it can occur beneath apparently intact surfaces, making it difficult to detect until severe damage has occurred. Stainless steels, aluminum alloys, and other passive metals are especially susceptible to crevice corrosion when their protective oxide film is compromised in these oxygen-starved environments.
Common prevention methods include designing joints to minimize tight gaps, using non-absorbent gaskets, selecting corrosion-resistant alloys, and applying protective coatings or sealants. Maintaining clean surfaces and avoiding stagnant conditions also help reduce risk.
In summary, crevice corrosion occurs when oxygen-starved microenvironments form within tight spaces on metal surfaces, leading to localized chemical attack that can severely weaken fasteners and assemblies over time.
Electrochemical Corrosion
Electrochemical corrosion is a type of metallic corrosion that occurs when a metal deteriorates through an electrochemical reaction involving anodic oxidation and cathodic reduction—essentially forming a tiny battery or galvanic cell on the metal’s surface. This process takes place when a metal, an electrolyte (like water with dissolved salts), and oxygen or another oxidizing agent are all present, allowing electrical current to flow between different areas of the metal.
In simple terms, corrosion happens because metals naturally want to return to their more stable, oxidized state, just like they exist in nature as ores. The electrochemical process allows this to occur through a redox reaction, where one area of the metal loses electrons (oxidation) and another gains them (reduction).
Here’s how it works:
1. Anodic reaction (oxidation):
At certain microscopic sites on the metal surface—called anodic regions—metal atoms lose electrons and dissolve into the surrounding electrolyte as metal ions. For example, in the case of iron:
Fe → Fe²⁺ + 2e⁻
This is where corrosion (metal loss) physically occurs.
2. Cathodic reaction (reduction):
The released electrons flow through the metal to another region—the cathode—where they are consumed by a reduction reaction, usually involving oxygen and water. In a neutral or basic environment, this is typically:
O₂ + 2H₂O + 4e⁻ → 4OH⁻
3. Electrolyte conduction:
The electrolyte (such as rainwater, seawater, or moisture on the surface) allows ionic movement between the anodic and cathodic areas, completing the electrochemical circuit.
4. Corrosion product formation:
The metal ions produced at the anode often react with oxygen or hydroxide ions in the electrolyte to form corrosion products—for example, iron oxide (rust) in steel.
Over time, this continuous cycle of oxidation and reduction leads to the gradual degradation of the metal. The process can be localized (as in pitting or crevice corrosion) or uniform, depending on environmental conditions, surface composition, and the presence of impurities or protective coatings.
Electrochemical corrosion is influenced by several factors, including:
- Electrochemical potential differences between microstructures or different metals.
- Electrolyte conductivity (higher salt content accelerates corrosion).
- Temperature and pH of the environment.
- Oxygen availability, which controls the cathodic reaction rate.
A classic example is galvanic corrosion, where two dissimilar metals (e.g., steel and copper) are electrically connected in a conductive environment. The more active metal (anode) corrodes faster, while the more noble metal (cathode) is protected.
To prevent electrochemical corrosion, engineers use methods such as protective coatings (paint, plating, galvanizing), cathodic protection (sacrificial anodes or impressed current systems), corrosion inhibitors, and material selection based on galvanic compatibility.
Galvanic Corrosion
Galvanic corrosion is a form of electrochemical corrosion that occurs when two different metals are in electrical contact in the presence of an electrolyte, such as water that contains salts, acids, or other conductive impurities. This situation creates a galvanic cell, where one metal functions as the anode and the other as the cathode. The anode is the more active metal, meaning it has a greater tendency to give up electrons, and therefore it corrodes more quickly than it would on its own. The cathode, being the more noble metal, is protected from corrosion. A common example is seen when steel and copper are connected in a marine environment: the steel, acting as the anode, corrodes rapidly, while the copper, as the cathode, remains unharmed.

The severity of galvanic corrosion is influenced by several conditions. The greater the difference in electrochemical potential between the two metals, the faster corrosion will occur. The conductivity of the electrolyte also plays a major role, with saltwater being particularly aggressive in accelerating the process. Additionally, the relative size of the metals in contact affects the outcome; a small anode connected to a large cathode will corrode at a much faster rate due to the disproportionate distribution of current.
To prevent or reduce galvanic corrosion, engineers employ several strategies. Metals that are close to each other in the galvanic series are often chosen to minimize potential differences. Coatings, insulators, or paints can be applied to separate the metals electrically. Protective measures such as galvanization or the use of sacrificial anodes, like zinc on ship hulls, can redirect corrosion to a controlled, replaceable material. Designers may also consider the ratio of exposed metal areas to reduce the imbalance between anodic and cathodic surfaces.
In fasteners and construction, galvanic corrosion is especially important to address because it can compromise the integrity of joints and assemblies. A classic example is the use of stainless steel bolts in aluminum structures. Without proper insulation or coatings, the aluminum can corrode quickly in a moist environment, weakening the structure and leading to premature failure. This makes galvanic corrosion a critical factor in selecting and designing fastening systems for long-term durability.
Pitting Corrosion
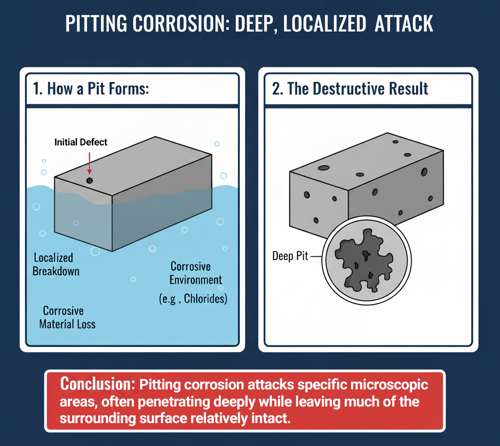
Pitting corrosion is a localized and highly destructive form of electrochemical corrosion that produces small, deep holes or “pits” on a metal surface. Unlike uniform corrosion—which affects a surface evenly—pitting attacks specific microscopic areas, often penetrating deeply while leaving much of the surrounding surface relatively intact. This makes it especially dangerous and difficult to detect, since severe structural damage can occur beneath an otherwise smooth surface.
Pitting corrosion typically begins when a protective oxide film (the passive layer) that normally shields metals such as stainless steel, aluminum, titanium, or nickel alloys is locally damaged or weakened. This can happen due to chloride ions (from saltwater or perspiration), mechanical scratches, chemical impurities, or stagnant fluid conditions. Once the film is breached, a tiny anodic site forms where metal dissolution begins:
Anodic reaction:
Fe → Fe²⁺ + 2e⁻
The nearby intact surface acts as a cathode, where a reduction reaction consumes the released electrons:
Cathodic reaction:
O₂ + 2H₂O + 4e⁻ → 4OH⁻
Inside the pit, metal ions hydrolyze in the presence of water, creating acidic conditions (low pH) that further accelerate dissolution. Meanwhile, chloride ions migrate into the pit, maintaining electrical neutrality and promoting even more localized attack. The chemistry inside the pit becomes self-sustaining, causing it to deepen rapidly while the rest of the surface remains passive.
This autocatalytic process can lead to narrow, deep cavities that compromise the structural integrity of thin components like tubes, tanks, or fasteners. Because pits can penetrate metal walls without significant overall mass loss, pitting corrosion is among the most insidious and dangerous forms of corrosion, often leading to sudden leakage or failure with little visible warning.
Common causes include:
- Chloride-containing environments, such as seawater or road salts.
- Poor oxygenation, which prevents passive film repair.
- Deposits or crevices that trap corrosive agents.
- Improper alloy selection or surface contamination during fabrication.
Prevention and control of pitting corrosion rely on:
- Using more resistant alloys (e.g., 316 stainless steel instead of 304, due to its molybdenum content).
- Maintaining clean, oxygenated surfaces so the passive film can regenerate.
- Applying protective coatings or cathodic protection.
- Avoiding chloride-rich environments when possible or using corrosion inhibitors.
Uniform Corrosion
Uniform corrosion is the most common and predictable form of metallic corrosion, characterized by the even and consistent attack of a metal surface over a large area. In this process, the corrosion reaction occurs uniformly across the exposed surface, causing the metal to thin gradually and evenly rather than developing localized pits or cracks.
This type of corrosion happens when the entire surface of the metal is exposed to a corrosive environment—for example, air, moisture, acids, or salts—and when the chemical or electrochemical conditions are consistent across that surface. Each microscopic area of the metal alternates between acting as an anode (where metal atoms oxidize and dissolve) and a cathode (where reduction reactions occur), resulting in an overall uniform rate of material loss.

A classic example is the rusting of unprotected carbon steel exposed to air and moisture, which produces iron oxides (Fe₂O₃) or hydrated rust evenly across the surface. Over time, this leads to surface roughening, scaling, and uniform wall thinning, which can weaken structural components like pipes, tanks, or machinery parts.
The general electrochemical reactions for uniform corrosion of iron in the presence of oxygen and water are:
- Anodic reaction: Fe → Fe²⁺ + 2e⁻
- Cathodic reaction: O₂ + 2H₂O + 4e⁻ → 4OH⁻
The resulting Fe²⁺ ions combine with hydroxide ions to form Fe(OH)₂, which oxidizes further into rust (Fe₂O₃·xH₂O).
Uniform corrosion rates can be measured and predicted relatively easily by weight loss tests, electrochemical methods, or thickness measurements. The rate depends on environmental factors such as humidity, temperature, pH, oxygen concentration, and the presence of salts or pollutants.
Although it is the least dangerous type of corrosion in terms of sudden failure—because the material loss is gradual and visible—it can still cause significant structural weakening over time if left untreated.
Prevention typically involves:
- Protective coatings (paints, platings, or galvanizing) to isolate the metal from the environment.
- Corrosion inhibitors added to liquids or systems.
- Cathodic protection, particularly for buried or submerged structures.
- Material selection, using corrosion-resistant metals or alloys such as stainless steel, aluminum, or titanium.
