Resources
Glossary
Electroplating (Plating)
Electroplating is a metal finishing process that uses electric current to deposit a thin layer of metal onto the surface of another material—usually metal, but sometimes plastic or other conductive substrates. The main goals of electroplating are to improve corrosion resistance, enhance appearance, reduce friction, increase hardness, or provide electrical conductivity.
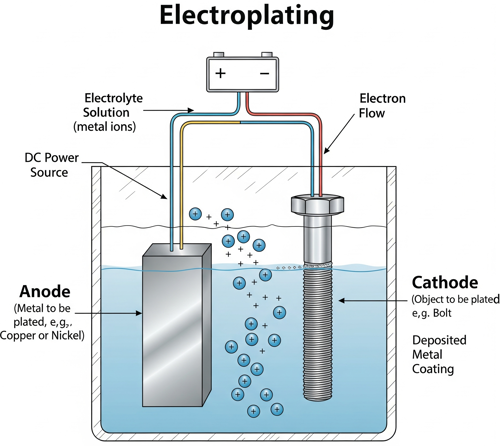
The process works through electrochemical deposition. The part to be plated (called the cathode) is submerged in a solution containing dissolved metal ions from the plating material, while another piece of the plating metal (the anode) is also immersed in the same solution. When direct current (DC) is applied, metal ions in the solution are attracted to and deposit onto the cathode, forming a uniform, tightly bonded metal coating.
For example, in zinc electroplating, a steel bolt is placed in a solution of zinc sulfate. When electricity flows, zinc ions are reduced and bond to the steel surface, forming a corrosion-resistant zinc coating.
The thickness and quality of the electroplated layer can be controlled by adjusting factors such as current density, plating time, temperature, and solution composition. The resulting finish can range from bright and decorative (like chrome or nickel) to functional and protective (like zinc, copper, or tin).
Common metals used in electroplating include:
- Zinc – for corrosion protection on steel.
- Nickel – for hardness and a smooth, bright finish.
- Chromium – for durability, luster, and wear resistance.
- Copper – for electrical conductivity and as an underlayer in multi-metal coatings.
- Gold and silver – for conductivity, aesthetics, and corrosion resistance in electronics.
AKA: Electro-Galvanizing
