Resources
Glossary
Absolute Temperature
Absolute temperature is temperature measured from absolute zero, the theoretical point where a system has its minimum possible thermal energy. The key feature is that an absolute temperature scale cannot go negative—because you can’t have “less than absolute zero” in the normal thermodynamic sense.
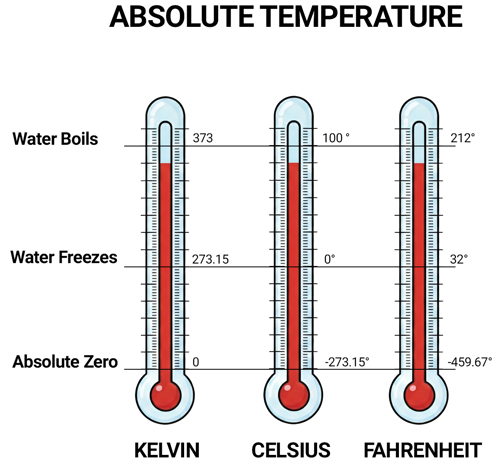
In industrial engineering and physics, absolute temperature matters because many core relationships depend on the true thermodynamic temperature, not a relative scale like °C or °F. For example, gas laws and many material/heat-transfer models use absolute temperature directly (so doubling T has real physical meaning only when T is absolute).
The SI (International System of Units) absolute temperature scale is Kelvin (K), where 0 K = absolute zero and K = °C + 273.15. In U.S. customary engineering, the Fahrenheit-sized absolute scale is Rankine (°R), where 0 °R = absolute zero and °R = °F + 459.67.
